Connecticut participates in nationwide effort to expand Medicaid coverage for expensive sickle cell gene therapies
- Last update: 19 minutes ago
- 3 min read
- 470 Views
- US
Advances in cell and gene therapies are providing groundbreaking treatment options for individuals with sickle cell disease. However, awareness of these therapies remains limited, and access is often hindered by their multi-million-dollar cost. Currently, fewer than 100 people in the U.S. have received gene therapy for this condition, according to Dr. Lakshmanan Krishnamurti, chief of pediatric hematology, oncology, and bone marrow transplant at Yale New Haven Children's Hospital.
To broaden access, Connecticut has joined 33 other states, Washington, D.C., and Puerto Rico in a pioneering federal initiative to make these therapies available through Medicaid. Collectively, these regions represent 84% of Medicaid recipients diagnosed with sickle cell disease.
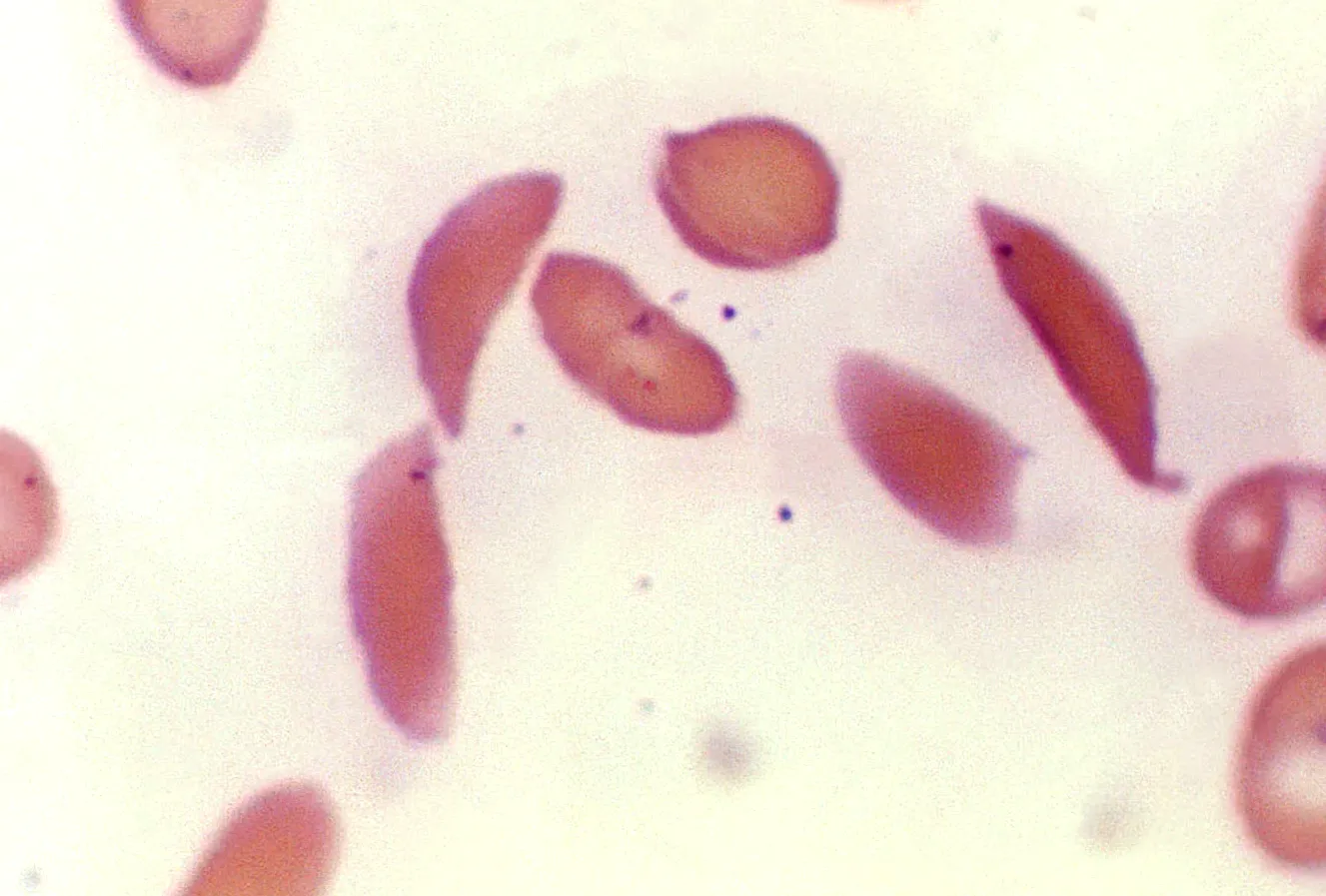
Approximately 100,000 Americans live with sickle cell disease, a hereditary blood disorder that predominantly affects people of color. Over 90% of patients are Black, and 3% to 9% are Latino, according to the Centers for Disease Control and Prevention. The disease is caused by a mutation in hemoglobin, the protein responsible for transporting oxygen in red blood cells. This mutation results in crescent-shaped, fragile cells that break down quickly and block blood vessels, leading to anemia and reduced oxygen delivery throughout the body.
Dr. Krishnamurti explained, "It's like freezing a balloon filled with waterit becomes rigid and breaks. Thats what happens to red blood cells in sickle cell disease."
The severity and symptoms of sickle cell disease vary, potentially causing chronic pain, infections, acute chest syndrome, stroke, and other serious complications. Chronic pain can significantly impair education, employment, and overall quality of life, reducing life expectancy by an estimated 20 years.
Traditional treatment relied on bone marrow transplants, which carry risks such as infertility and immune rejection. Daily oral medications are increasingly common but require lifelong adherence and do not fully prevent premature mortality.
Gene therapy offers a transformative approach. By reprogramming a patients own blood cells to produce healthy hemoglobin and then reinfusing them, these therapies can fundamentally alter disease outcomes. Currently, the FDA has approved two treatments for patients aged 12 and older: Casgevy by Vertex Pharmaceuticals and Lyfgenia by Bluebird Bio.
Casgevy modifies a patients stem cells to boost fetal hemoglobin production, while Lyfgenia uses engineered stem cells delivered via a modified virus to generate functional hemoglobin. Both treatments are available at Yale and require a comprehensive, year-long process including stem cell collection, chemotherapy, transfusions, monitoring, and psychological rehabilitation.
The cost of Casgevy is $2.2 million, and Lyfgenia is $3.1 million. Despite the high price, Dr. Krishnamurti notes that the total lifetime care for a patient with sickle cell disease can exceed these amounts, making gene therapy a potentially cost-effective investment.
The federal Cell and Gene Therapy Access Model aims to test whether outcome-based agreements can expand treatment access, improve patient outcomes, and reduce state healthcare costs. Under this model, drug prices are tied to effectiveness, with participating states eligible for rebates if treatments fail to meet expected results.
Connecticut is among seven states receiving additional federal support to cover program costs and ensure equitable access for underserved communities. The states Department of Social Services expects to finalize agreements and launch the program by January 1.
"Gene therapy offers the possibility of living fully without continuous medication or limitations," said Dr. Krishnamurti. "Patients should consult their hematologist to explore if this option is right for them."
Author: Ava Mitchell